Using the Beer Lambert Law to Calculate Concentration
The proportionality constant of the equation is termed as the molar extinction coefficient of the substance. A spectrophotometer value detected A070.

Spectrophotometry And Beer Lambert Law Youtube
This video shows how to use the Beer-Lambert law to calculate the extinction coefficient ε for a sample when the absorbance concentration and path length.

. Calculate the concentration of the dye using Beer-Lamberts law if the absorption at 630nm is 125 the optical path length b is 10cm and the molar absorptivity coefficient is 138x105 M-1cm-1. Divide the mass of the solute by the total volume of the solution. Calculate the length of the path in which the light beam travels.
This calculator can be used to find the amount light absorbed by the medium it is passing through. This video shows how to use the Beer-Lambert law to calculate the concentration c for a sample when the absorbance extinction coefficient and path length. According to Beer-Lambert law log Io It A.
What does the Beer Lambert law state. The search for results was accompanied by a mathematical calculation of approximating linear model construction of calibration straight. The BeerLambert law relates the absorption of light by a solution to the properties of the solution according to the following equation.
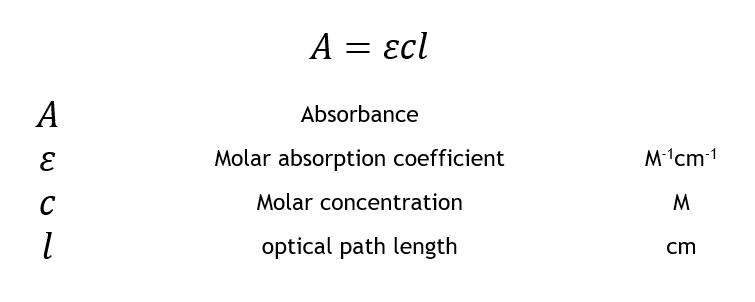
A εbc where ε is the molar absorptivity of the absorbing species b is the path length and c is the concentration of the absorbing species. Using the Beer-Lambert Law to Calculate the Concentration of a Solution High School Chemistry Skills Practice Retinol is a vitamin that can be detected using UV spectroscopy. Search Results related to calibration curve and.
Finding concentration of unknown solution using Beer-Lamberts law. The formula sets absorbance equal to extinction coefficient molar absorptivity in M-1 cm-1 multiplied by path length and concentration. The BeerLambert law relates the absorption of light by a solution to the properties of the solution according to the following equation.
By using Beers law we will calculate the concentration of the sample. A εbc where ε is the molar absorptivity of the absorbing species b is the path length and c is the concentration of the absorbing species. A εbc where ε is.
Use the Beer-Lambert Law to calculate concentration given the following information. Write out the equation C mV where m is the mass of the solute and V is the total volume of the solution. Find the concentration of the solution.
According to the Beer Lambert Law the Absorbance is proportional to the path length distance that light travels through the material and the concentration of the material. The path length is 1 cm and the concentration is. Concentration was created using Excel by using the increasing concentrations of the five standard solutions for the x values and their corresponding absorbances for the y values.
C l or c A. Calculate the concentration of the sample. Write out the equation C mV where m is the mass of the solute and V is the total volume of the solution.
Dye mL 20 Vol H2O mL 3 Vol NaOCl mL 3 Time s 195. Plug in the values you found for the mass and volume and divide them to find the concentration of your solution. We can now the transmittance plug into the absorbance formula A -log 084 0076 Our absorbance value is 0076.
L whereIoand Itare the incident and transmitted intensitiesA absorbance and a constant absorptivity formerly called the extinction coefficientIf the concentration is. A calibration curve displaying Absorbance vs. In this report it was explained how the use of the Beer-Lambert spectroscopic law allows determining the concentration of the active ingredient in medicinal products which moreover was the central purpose of the work.
How to calculate concentration using the Beer Lambert law. Calculate the rate of reaction given the following data. In Part 2 a small amount of Cola was heated in a beaker covered with a watch glass to reduce evaporation.
Steps to calculate absorption from Beer-Lambert Law Step 1. A εbc where ε is the molar absorptivity of the absorbing species b is the path length and c is the concentration of the absorbing species. Contents hide 1 How does Beers law relate to concentration.
Divide the mass of the solute by the total volume of the solution. Determine the molar absorption coefficient of the solution. It is possible to measure the Beer Lambert law by calculating the concentration of a solution by making use of the absorbancies.
This is a calculator to find a missing Beer Lambert Law equation value. The Beer-Lambert law relates the absorption of light by a solution to the properties of the solution according to the following equation. The molar absorptive is 120 L mol cm and the path length is 10 mm.
Plug in the values you found for the mass and volume and divide them to find the concentration of your solution. Afterwards one must use a colourimeter to calculate the concentration of an unknown solution. A εbc 070 8400 M-1cm-1 1cm c By dividing both sides of the equation by 8400 M-1cm-1 1cm c 833 x 10-5 molL Transmittance and absorbance of light using Beers law.
The absorbance of a sample is 016. Another way is to plot a graph of various concentrations and then align them according to their appropriate or correct absorbencies. Contents hide 1 How do you use Beers Law.
The BeerLambert law relates the absorption of light by a solution to the properties of the solution according to the following equation.
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments

Using The Beer Lambert Law To Calculate The Concentration Of A Solution Chemistry Study Com

How To Find Molar Absorptivity Using The Beer Lambert Law Chemistry Study Com
Comments
Post a Comment